Holozoa
Holozoa is a group of organisms that includes animals and their closest single-celled (protist) relatives, but excludes fungi and all other organisms. It is a monophyletic group or clade, a lineage consisting of all descendants of a common ancestor. Among these descendants, the protists are of high interest because of their close relationship to animals: in the search for the genes responsible for animal multicellularity within these protists, they help elucidate the nature of the unicellular ancestor of animals.[5][6][7][8]
| Holozoans Temporal range: | |
|---|---|
 | |
| Scientific classification | |
| Clade: | Amorphea |
| Clade: | Obazoa |
| (unranked): | Opisthokonta |
| (unranked): | Holozoa Lang et al., 2002[1] |
| Subgroups[2] | |
| |
Definition and subdivisions
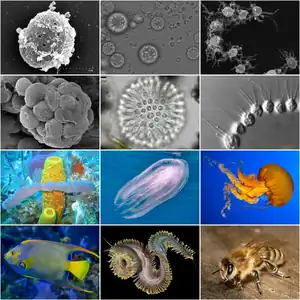
Holozoa is the most inclusive clade containing Homo sapiens (a metazoan), but not Neurospora crassa (a fungus). It is a clade with a branch-based definition: it contains all the closest relatives to animals that aren't fungi, as well as their common ancestor.[2] The clade was first discovered through phylogenetic analyses in 2002.[1] These mostly unicellular relatives are the protist lineages of choanoflagellates, filastereans, ichthyosporeans, and three independent species Corallochytrium, Syssomonas[9] and Tunicaraptor.[3]
- Choanoflagellata (>250 species)[10] are the protists most closely related to animals. They are free-living unicellular or colonial flagellates that feed on bacteria using a characteristic “collar” of microvilli. This collar strongly resembles the collar cells of sponges;[11] because of this, choanoflagellates were theorized to be related to sponges even in the 19th century. The mysterious Proterospongia is an example of a colonial choanoflagellate that was thought to be related to the origin of sponges.[12] The affinities of the other single-celled holozoans only began to be recognized in the 1990s.[13]
- Ichthyosporea or Mesomycetozoea (~40 species) are mostly parasites or commensals of a wide variety of animals, including humans, fish and marine invertebrates. Most reproduce through multinucleated colonies and disperse as flagellates or amoebae.[10]
- Filasterea is a group composed by the amoeboid genera Ministeria, Pigoraptor[9] and Capsaspora, united by the structure of their thread-like pseudopods.[7]
- Pluriformea is a group composed by the genera Corallochytrium and Syssomonas.[9]
- Tunicaraptor is a recently discovered lineage whose position within Holozoa has yet to be resolved.[3]
Evolutionary history
Holozoan phylogeny
Based on phylogenetic and phylogenomic analyses, the cladogram of Holozoa is shown below, with indications of the time divergence of the different clades in millions of years (Mya).[14][15][9][3] The choanoflagellates, animals and filastereans group together as the clade Filozoa.[7] The Pluriformea clade (Corallochytrium and Syssomonas) is the sister group to Filozoa. An alternative hypothesis is the Teretosporea clade, which unites Pluriformea with Ichthyosporea instead.[9]
| Opisthokonta |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1300 Mya |
Unicellular ancestor of animals
The search for the origin of animals, or the nature of the last metazoan common ancestor (or Urmetazoan), requires deciphering the unicellular-to-multicellular transition that took place. Due to the absence of a fossil record, the only way to investigate the nature of the unicellular metazoan common ancestor and its initial steps towards multicellularity is by studying the genes and genetic pathways shared between animals and their closest living relatives.[10]
The sequencing of unicellular holozoan genomes allows us to reconstruct the gene content of this unicellular metazoan ancestor with a high level of detail.[10] Many traits previously considered “specific to animals” are found in their unicellular relatives,[9] indicating that multicellularity appeared not through the acquisition of new genes exclusive to animals, but through the co-option of ancestral genes already present in their unicellular relatives.[10] For example:
- Genes encoding adhesion proteins are necessary for cell-cell and cell-matrix adhesion in the formation of layers, tissues and the extracellular matrix (ECM) in animals. Choanoflagellates have C-type lectins, involved in cell-cell adhesion, and proteins containing a cadherin domain, although the β-catenin that regulates animal cadherins seems to be specific to animals. The filasterean Capsaspora has a complete integrin adhesome, a major cell-ECM adhesion system in animals.[10] Other holozoans have ECM-related proteins, including components of the dystrophin-associated protein complex, laminins, collagens and fibronectins.[16]
- Signal transduction genes are another requirement for metazoan multicellularity. Developmental signaling pathways that are highly conserved in metazoans (such as Hedgehog, WNT, TGFβ, JAK-STAT and Notch) are absent in non-metazoans. Similar but non-homologous signaling receptors appear in unicellular holozoans, such as the receptor tyrosine kinases, which evolved independently in choanoflagellates, filastereans and ichthyosporeans. However, some animal cytoplasmic tyrosine kinases (such as focal adhesion kinase) and the Hippo signaling pathway are present in unicellular holozoans.[10]
- A considerable portion of animal transcription factors (TF) is already present in unicellular holozoans, including some TF classes previously thought to be animal-specific (such as p53 and T-box). Other classes and families are animal-specific, such as SMAD, Doublesex and IRF.[10]
Additionally, many biological processes seen in animals were already present in their unicellular ancestor, such as sexual reproduction and gametogenesis in the choanoflagellate Salpingoeca rosetta and several types of multicellular differentiation.[10]
Fossil record

A billion-year-old freshwater microscopic fossil known as Bicellum brasieri is possibly a holozoan that shows two differentiated cell types or life cycle stages. It consists of a spherical ball of tightly packed cells (stereoblasts) enclosed in a single layer of elongated cells. There are also two populations of stereoblasts with mixed shapes, which have been interpreted as cellular migration to the periphery, a movement that could be explained by differential cell-cell adhesion. These occurrences are consistent with extant unicellular holozoans, which are known to form multicellular stages in complex life cycles.[4]
Proposed Ediacaran fossil "embryos" of early metazoans, discovered in the Doushantuo formation, have been reinterpreted as non-animal protists within Holozoa. According to some authors, although they present possible embryonic cleavage, they lack metazoan synapomorphies such as tissue differentiation and nearby juveniles or adults. Instead, its development is comparable to the germination stage of non-animal holozoans. They possibly represent an evolutionary grade in which palintomic cleavage (i.e. rapid cell divisions without cytoplasmic growth in between, a characteristic of animal embryonic cleavage)[17] was the method of dispersal and propagation.[18]
Taxonomy
Because Holozoa is a clade including animals and all protists more closely related to animals than to fungi, some authors prefer it as opposed to recognizing paraphyletic groups that mostly consists of Holozoa minus animals,[19] such as the paraphyletic phylum “Choanozoa” adopted by the protozoologist Thomas Cavalier-Smith.[lower-alpha 1] The International Society of Protistologists prefers classifying eukaryotes in monophyletic groups, striving away from traditional ranks (kingdom, phylum, etc.). According to this view, the classification of Holozoa is:[2]
- Holozoa Lang et al. 2002
- †Bicellum brasieri Strother & Wellman 2021[4]
- Tunicaraptor Tikhonenkov, Mikhailov, Hehenberger, Karpov, Prokina, Esaulov, Belyakova, Mazei, Mylnikov, Aleoshin & Keeling 2020[3]
- Ichthyosporea[lower-alpha 2] Cavalier-Smith 1998 [Mesomycetozoea Mendoza et al. 2002]
- Dermocystida Cavalier-Smith 1998 [Rhinosporidaceae Mendoza et al. 2001]
- Ichthyophonida Cavalier-Smith 1998 [Ichthyophonae Mendoza et al. 2001; Amoebidiidae Reeves 2003]
- Pluriformea[lower-alpha 2] Hehenberger et al. 2017
- Corallochytrium limacisporium Raghu-Kumar 1987
- Syssomonas multiforma[lower-alpha 2] Tikhonenkov, Hehenberger, Mylnikov & Keeling 2017
- Filasterea Shalchian-Tabrizi et al. 2008
- Capsaspora owczarzaki Hertel, Bayne & Loker, 2002
- Ministeria Patterson et al. 1993
- Pigoraptor Tikhonenkov et al. 2017
- Txikispora Urrutia, Feist & Bass 2022[20]
- Choanozoa Brunet & King 2017 [Choanozoa Cavalier-Smith et al. 1991 (P)][lower-alpha 1]
- Choanoflagellata Kent 1880–1882 [Craspedomonadina Stein 1878; Craspedomonadaceae Senn 1900; Craspedophyceae Chadefaud 1960; Craspédomonadophycidées Bourrelly 1968; Craspedomonadophyceae Hibberd 1976; Choanomonadea Krylov et al. 1980; Choanoflagelliida Lee, Hutner & Bovee 1985; Choanoflagellatea Cavalier-Smith 1997 emend. Cavalier-Smith 1998; Choanomonada Adl et al. 2005]
- Craspedida Cavalier-Smith 1997, emend. Nitsche et al. 2011
- Salpingoecidae Kent 1880–1882, emend. sensu Nitsche et al. 2011
- Acanthoecida Cavalier-Smith 1997, emend. Nitsche et al. 2011
- Acanthoecidae Norris 1965, emend. sensu Nitsche et al. 2011
- Stephanoecidae Leadbeater 2011
- Craspedida Cavalier-Smith 1997, emend. Nitsche et al. 2011
- Metazoa Haeckel 1874, emend. Adl et al. 2005 [Animalia Linnaeus 1758; Eumetazoa Bütschli 1910]
- Porifera Grant 1836 [Parazoa Sollas 1884]
- Placozoa Grell 1971
- Ctenophora Eschscholtz 1829
- Cnidaria Hatschek 1888
- Bilateria Hatschek 1888
- Choanoflagellata Kent 1880–1882 [Craspedomonadina Stein 1878; Craspedomonadaceae Senn 1900; Craspedophyceae Chadefaud 1960; Craspédomonadophycidées Bourrelly 1968; Craspedomonadophyceae Hibberd 1976; Choanomonadea Krylov et al. 1980; Choanoflagelliida Lee, Hutner & Bovee 1985; Choanoflagellatea Cavalier-Smith 1997 emend. Cavalier-Smith 1998; Choanomonada Adl et al. 2005]
Notes
- The term "Choanozoa" has been used since 1991 by Cavalier-Smith as a paraphyletic phylum of opisthokont protists,[21] and the terms "Apoikozoa" and "choanimal" were proposed as names for the clade Metazoa+Choanoflagellata. However, these terms have not been formally described or adopted, and were rejected in favour of a renamed Choanozoa to fit the clade Metazoa+Choanoflagellata.[2]
- There are two competing phylogenetic hypotheses: Teretosporea (Corallochytrium + Ichthyosporea) and Pluriformea (Corallochytrium + Syssomonas).[2] This article follows the latter hypothesis, because it is strongly supported by the most recent phylogenetic studies.[3]
References
- Lang BF, O'Kelly C, Nerad T, Gray MW, Burger G (2002). "The Closest Unicellular Relatives of Animals". Current Biology. 12 (20): 1773–1778. doi:10.1016/S0960-9822(02)01187-9.
- Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov A, Agatha S, Berney C, Brown MW, Burki F, Cárdenas P, Čepička I, Chistyakova L, del Campo J, Dunthorn M, Edvardsen B, Eglit Y, Guillou L, Hampl V, Heiss AA, Hoppenrath M, James TY, Karnkowska A, Karpov S, Kim E, Kolisko M, Kudryavtsev A, Lahr DJG, Lara E, Le Gall L, Lynn DH, Mann DG, Massana R, Mitchell EAD, Morrow C, Park JS, Pawlowski JW, Powell MJ, Richter DJ, Rueckert S, Shadwick L, Shimano S, Spiegel FW, Torruella G, Youssef N, Zlatogursky V, Zhang Q (2019). "Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes". Journal of Eukaryotic Microbiology. 66 (1): 4–119. doi:10.1111/jeu.12691. PMC 6492006. PMID 30257078.
- Tikhonenkov DV, Mikhailov KV, Hehenberger E, Mylnikov AP, Aleoshin VV, Keeling PJ, et al. (2020). "New Lineage of Microbial Predators Adds Complexity to Reconstructing the Evolutionary Origin of Animals". Current Biology. 30 (22): 4500–4509. doi:10.1016/j.cub.2020.08.061. PMID 32976804.
- Strother, Paul K.; Brasier, Martin D.; Wacey, David; Timpe, Leslie; Saunders, Martin; Wellman, Charles H. (April 2021). "A possible billion-year-old holozoan with differentiated multicellularity". Current Biology. 31 (12): 2658–2665.e2. doi:10.1016/j.cub.2021.03.051. PMID 33852871.
- Aleshin, V.V. (December 2007). Konstantinova, A.V.; Mikhailov, K.V.; Nikitin, M.A.; Petrov, N.B. "Do we need many genes for phylogenetic inference?". Biochemistry Mosc. 72 (12): 1313–23. doi:10.1134/S000629790712005X. PMID 18205615. S2CID 12594007.
- Lang, B.F. (October 2002). O'Kelly, C., Nerad, T., Gray, M.W., Burger, G. "The closest unicellular relatives of animals". Current Biology. 12 (20): 1773–8. doi:10.1016/S0960-9822(02)01187-9. PMID 12401173. S2CID 14192333.
- Shalchian-Tabrizi, Kamran; Minge, Marianne A.; Espelund, Mari; Orr, Russell; Ruden, Torgeir; Jakobsen, Kjetill S.; Cavalier-Smith, Thomas; Aramayo, Rodolfo (7 May 2008). Aramayo, Rodolfo (ed.). "Multigene phylogeny of choanozoa and the origin of animals". PLOS ONE. 3 (5): e2098. Bibcode:2008PLoSO...3.2098S. doi:10.1371/journal.pone.0002098. PMC 2346548. PMID 18461162.
- Elias, M.; Archibald, J.M. (August 2009). "The RJL family of small GTPases is an ancient eukaryotic invention probably functionally associated with the flagellar apparatus". Gene. 442 (1–2): 63–72. doi:10.1016/j.gene.2009.04.011. PMID 19393304.
- Hehenberger, Elisabeth; Tikhonenkov, Denis V.; Kolisko, Martin; Campo, Javier del; Esaulov, Anton S.; Mylnikov, Alexander P.; Keeling, Patrick J. (2017). "Novel Predators Reshape Holozoan Phylogeny and Reveal the Presence of a Two-Component Signaling System in the Ancestor of Animals". Current Biology. 27 (13): 2043–2050.e6. doi:10.1016/j.cub.2017.06.006. PMID 28648822.
- Sebé-Pedrós A, Degnan B, Ruiz-Trillo I (2017). "The origin of Metazoa: a unicellular perspective". Nature Reviews Genetics. 18: 498–512. doi:10.1038/nrg.2017.21.
- Simpson AGB, Slamovits CH, Archibald JM (2017). "Chapter 1. Protist Diversity and Eukaryote Phylogeny". In Archibald JM, Simpson AGB, Slamovits CH (eds.). Handbook of the Protists. Vol. 1 (2 ed.). Springer International Publishing. pp. 1–22. ISBN 978-3-319-28147-6.
- Brunet T, King N (2022). "The Single-Celled Ancestors of Animals: A History of Hypotheses". In Herron MD, Conlin PL, Ratcliff WC (eds.). The Evolution of Multicellularity. Evolutionary Cell Biology. CRC Press. pp. 251–278. doi:10.1201/9780429351907-17. ISBN 9780429351907.
- Ragan, Mark A.; Goggin, C. Louise; Cawthorn, Richard J.; Cerenius, Lage; Jamieson, Angela V.C.; Plourde, Susan M.; Rand, Thomas G.; Söoderhäll, Kenneth; Gutell, Robin R. (15 October 1996). "A novel clade of protistan parasites near the animal-fungal divergence". PNAS. 93 (21): 11907–11912. Bibcode:1996PNAS...9311907R. doi:10.1073/pnas.93.21.11907. PMC 38157. PMID 8876236.
- Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (August 16, 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–13629. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- Torruella, Guifré; de Mendoza, Alex; Grau-Bové, Xavier; Antó, Meritxell; Chaplin, Mark A.; del Campo, Javier; Eme, Laura; Pérez-Cordón, Gregorio; Whipps, Christopher M. (21 September 2015). "Phylogenomics Reveals Convergent Evolution of Lifestyles in Close Relatives of Animals and Fungi". Current Biology. 25 (18): 2404–2410. doi:10.1016/j.cub.2015.07.053. ISSN 0960-9822. PMID 26365255.
- Suga H, Chen Z, de Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, Carr M, Kerner P, Vervoot M, Sánchez-Pons N, Torruella G, Derelle R, Manning G, Lang BF, Russ C, Haas BJ, Roger AJ, Nusbaum C, Ruiz-Trillo I (2013). "The Capsaspora genome reveals a complex unicellular prehistory of animals". Nature Communications. 4 (2325). doi:10.1038/ncomms3325.
- Chen L, Xiao S, Pang K, Zhou C, Yuan X (September 2014). "Cell differentiation and germ–soma separation in Ediacaran animal embryo-like fossils". Nature. 516: 238–241. doi:10.1038/nature13766.
- Huldtgren T, Cunningham JA, Yin C, Stampanoni M, Marone F, Donoghue PCJ, Bengtson S (2011). "Fossilized Nuclei and Germination Structures Identify Ediacaran "Animal Embryos" as Encysting Protists". Science. 334 (6063): 1696–1699. doi:10.1126/science.1209537.
- Steenkamp, Emma T.; Wright, Jane; Baldauf, Sandra L. (January 2006). "The Protistan Origins of Animals and Fungi". Molecular Biology and Evolution. 23 (1): 93–106. doi:10.1093/molbev/msj011. PMID 16151185.
- Urrutia A, Mitsi K, Foster R, Ross S, Carr M, Ward GM, et al. (2022). "Txikispora philomaios n. sp., n. g., a micro-eukaryotic pathogen of amphipods, reveals parasitism and hidden diversity in Class Filasterea". Journal of Eukaryotic Microbiology. 69 (2): e12875. doi:10.1111/jeu.12875. PMID 34726818. S2CID 240422937.
- Cavalier-Smith T (May 2013). "Early evolution of eukaryote feeding modes, cell structural diversity, and classification of the protozoan phyla Loukozoa, Sulcozoa, and Choanozoa". European Journal of Protistology. 49 (2): 115–178. doi:10.1016/j.ejop.2012.06.001.