Nerve compression syndrome
Nerve compression syndrome, or compression neuropathy, or nerve entrapment syndrome, is a medical condition caused by chronic, direct pressure on a peripheral nerve.[1] It is known colloquially as a trapped nerve, though this may also refer to nerve root compression (by a herniated disc, for example). Its symptoms include pain, tingling, numbness and muscle weakness. The symptoms affect just one particular part of the body, depending on which nerve is affected. The diagnosis is largely clinical and can be confirmed with diagnostic nerve blocks. Occasionally imaging and electrophysiology studies aid in the diagnosis. Timely diagnosis is important as untreated chronic nerve compression may cause permanent damage. A surgical nerve decompression can relieve pressure on the nerve but cannot always reverse the physiological changes that occurred before treatment. Nerve injury by a single episode of physical trauma is in one sense an acute compression neuropathy but is not usually included under this heading, as chronic compression takes a unique pathophysiological course.
| Nerve compression syndrome | |
|---|---|
| Other names | Entrapment neuropathy |
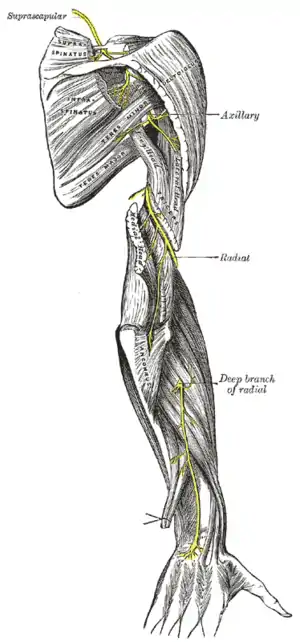 | |
| Radial nerve compression is an example of nerve compression syndrome | |
| Specialty | Neurology |
Syndromes
- Upper limb
| nerve | location | usually referred to as |
|---|---|---|
| Median | carpal tunnel | carpal tunnel syndrome |
| Median (anterior interosseous) | proximal forearm | anterior interosseous syndrome |
| Median | pronator teres | pronator teres syndrome |
| Median | ligament of Struthers | Ligament of Struthers syndrome |
| Ulnar | cubital tunnel | Cubital tunnel syndrome |
| Ulnar | Guyon's canal | Guyon's canal syndrome |
| Radial | axilla | Radial nerve compression |
| Radial | spiral groove | Radial nerve compression |
| Radial (Posterior interosseous) | proximal forearm | posterior interosseous nerve entrapment |
| Radial (Superficial radial) | distal forearm | Wartenberg's Syndrome |
| Suprascapular | Suprascapular canal | suprascapular nerve entrapment |
- Lower limb, abdomen and pelvis
| nerve | location | usually referred to as |
|---|---|---|
| Common peroneal | fibular neck | peroneal nerve compression |
| Tibial | tarsal tunnel | tarsal tunnel syndrome |
| Saphenous | Roof of Adductor canal | Saphenous nerve entrapment syndrome |
| Lateral cutaneous nerve of thigh | inguinal ligament | meralgia paraesthetica |
| Sciatic | piriformis | piriformis syndrome [not always due to entrapment] |
| Iliohypogastric | lower abdomen | iliohypogastric nerve entrapment |
| Obturator | obturator canal | obturator nerve entrapment |
| Pudendal | pelvis | pudendal nerve entrapment |
| Abdominal cutaneous nerves | abdominal wall | anterior cutaneous nerve entrapment syndrome |
| posterior femoral cutaneous | gluteal space | posterior femoral cutaneous nerve entrapment |
| middle cluneal | posterior sacroiliac ligament | middle cluneal nerve entrapment (MCN-E) |
| sacral plexus (s1-s4) | pelvis | sacral plexus entrapment or lumbosacral plexus entrapment |
| superior cluneal | posterior iliac crest | super cluneal nerve entrapment (SCN-E) |
Signs and symptoms
Symptoms vary depending on whether the affected nerve contains motor and/or sensory fibers. Sensory nerve entrapment presents with paresthesias. These paresthesias may be painful, such as shooting pain, burning, or a dull ache. They may also be pain-free, such as numbness or tingling. Motor nerve entrapment may present with muscle weakness or paralysis for voluntary movements of the innervated muscles. Entrapment of certain pelvic nerves can cause incontinence and/or sexual dysfunction.[2] Positive sensory symptoms are usually the earliest to occur, particularly tingling and neuropathic pain, followed or accompanied by reduced sensation or complete numbness. Muscle weakness is usually noticed later, and is often associated with muscle atrophy.
The distribution of symptoms is highly specific to the nerve entrapped and the way the nerve courses and branches beyond the entrapment point. For a given entrapment neuropathy, symptoms will only present in the areas innervated by that nerve and distal to the entrapment point. The symptom distribution is highly dependent on a patient's neuroanatomy, which may mean that two patients can present differently despite having the same nerve entrapped.
The timing/duration of symptoms may be continuous, intermittent, and/or positional. This is dependent on the underlying cause of entrapment and the specific nerves involved. For example, pain while sitting is associated with inferior cluneal nerve entrapment, pudendal nerve entrapment, and anococcyeal nerve entrapment.[3][4][5]
Causes
Certain occupations, postures, and activities can put prolonged pressure on a nerve. The term “Saturday night palsy” is used for a radial nerve injury caused by prolonged compression of the nerve at the spiral groove. The origin of the term is due to the association of the condition with a night spent in alcoholic stupor with the arm draped over a chair or bench. Mechanical compression of the radial nerve in the spiral groove can also occur as a result of the continuous use of crutches or prolonged kneeling in a “shooting” position.[6] The so-called "cyclist palsy" is caused by prolonged grip pressures on handlebars, and has been postulated to be an entrapment neuropathy of the ulnar nerve in the Guyon canal of the wrist.[7] Occupational exposure to forceful handgrip work and vibration, such as construction workers, increased the risk for surgical treatment of radial nerve entrapment.[8] Posture induced common peroneal nerve (CPN) palsy is usually produced during the prolonged squatting or habitual leg crossing while seated, especially in Asian culture and is manifested by the onset of foot drop.[9] One sport-related cause of lateral femoral cutaneous nerve entrapment is seen in scuba divers where the weight belt worn around the waist directly compresses the nerve.[10] Prolonged periods of cycling can be associated with pudendal nerve entrapment, as there is often direct compression on the pudendal nerve between the nose of the bicycle seat and pubic bone.[11]
Nerve compression can be secondary to other medical conditions. Entrapment neuropathies are remarkably common in diabetes.[12] A well defined lesion such as a tumor, hypertrophic muscle, cyst, hernia, hematoma, etc. can increase pressure on surrounding soft tissue, including nerves. Alternatively, there may be expansion of the tissues around a nerve in a space where there is little room for this to occur, as is often the case in carpal tunnel syndrome. This may be due to weight gain or peripheral oedema (especially in pregnancy), or to a specific condition such as acromegaly, hypothyroidism or scleroderma and psoriasis. There is increasing research that some forms of nerve entrapment, such as those in the hip/pelvis, can be secondary to abnormalities of the hip/spine leading to abnormal biomechanics.[13] With abnormal biomechanics, even normal postures and activities can put pressure on nerves.
Entrapment can be caused by injuries. Surgical injuries can cause entrapment by the development of scar tissue around the nerve as well as the decreased ability of the nerve to glide, increasing strain during movements. Radial nerve entrapment is seen after fracture manipulation when the nerve is unknowingly entrapped between bone and an installed plate, compressed by a bone fragment or if excessive nailing of the bone occurs.[14] Accidents are also associated with nerve entrapment as swelling puts pressure on the nerve and the development of scar tissue nearby may provide a hard surface for the nerve to be squeezed against.
Surgical and anatomic research has shed some light on the proximate causes of entrapment. There are anatomical regions in which segments of peripheral nerves are vulnerable or predisposed to become trapped and suffer from chronic compression. Neural compression occurs especially in osteofibrous tunnels but may also occur at points of passage of the peripheral nerve through the muscles or near a band of fibrous tissue.[15] In sciatic nerve decompression study, compromising structures were piriformis muscle, fibrovascular bundles, and adhesion with scar tissues.[16] In another endoscopic neurolysis study, the presence of fibrovascular bands and bursal tissue was the most common cause, followed by musculotendinous structures.[17]
Genetics may play a role in creating the necessary conditions for entrapment to occur. Previously, physicians thought repetitive wrist and hand motions were the only cause of carpal tunnel syndrome, especially in frequent computer users. But now doctors understand that the syndrome is probably a congenital predisposition in that some individuals have bigger carpal tunnels as compared to others.[18] Gene variants associated with musculoskeletal growth and extracellular matrix architecture have been implicated in carpal tunnel syndrome.[19] A rarer genetic cause is HNPP.
Pathophysiology
Acute and chronic compression of a nerve in a given area can lead to a cascade of physiological changes resulting in impaired function and then anatomical changes in the later stages.[20] Specifically, increased pressure on a nerve compresses the neural microvasculature and alters the blood flow dynamics.[21] Prolonged ischaemia and mechanical compromise may induce downstream effects such as inflammation, demyelination, scarring, and eventually axon degeneration. Neuroinflammation sensitizes injured and uninjured axons and nociceptors in target tissue, contributing to neuropathic pain initiation and maintenance. Focal demyelination is a hallmark of entrapment neuropathies, which are often characterized by nerve conduction slowing or block.[22] The initial changes are a break-down in the blood nerve barrier, followed by sub-perineurial edema and fibrosis; localized, then diffuse, demyelination occurs, and finally Wallerian degeneration.[23]
Animal models demonstrate that extraneural pressures as low as 20 to 30 mm Hg disrupt intraneural venous circulation. These pressures are often reached in patients with entrapment neuropathies. In several animal models, low magnitude, chronic nerve compression causes a biological response of: endoneurial edema, demyelination, inflammation, distal axon degeneration, extensive fibrosis, new axon growth, remyelination, and thickening of the perineurium and endothelium. Axonal degeneration was correlated with degree of endoneurial edema.[24]
In a few case reports (surgical resection of nerve, autopsy with known disease) the nerve at the site of injury was compared to a site proximal or distal to the injury. In each case, the site of injury demonstrated thickening of the walls of the microvessels in the endoneurium and perineurium along with epineurial and perineurial edema, thickening and fibrosis. Myelin thinning was also noted along with evidence of fiber degeneration and regeneration.[24] Experimental studies suggest a dose response curve such that the greater the duration and amount of pressure, the more significant is neural dysfunction.[23]
Diagnosis
Clinical diagnosis
Clinical diagnosis can often identify compression neuropathy on signs and symptoms alone. While there are variations in how nerves course and branch, the anatomical territory of major nerves do not change from patient to patient. Some forms of nerve entrapment can have characteristic symptoms, such as sitting and pudendal pain. Pudendal neuralgia, for example, is diagnosed by the Nantes criteria with four out of five criteria being clinical.[25]
Diagnostic nerve blocks
Diagnostic nerve blocks are very effective for identifying sensory entrapment points. Their strength is that they can directly measure whether a given nerve is contributing pain, or not. As successful blocks require accurate targeting of the nerve, this is done under image guidance such as fluoroscopy, ultrasound,[26] CT,[27] or MRI.[28] Ultrasound is popular choice because of its soft-tissue contrast, portability, lack of radiation, and low cost, but is not good at depicting deeper structures like the deep pelvic nerves. For deeper structures, CT and MRI are more appropriate, although they are more expensive and require higher operator skill.
The challenge with diagnostic blocks is that there often not good information to indicate exactly where the entrapment point may be. Consequently, multiple blocks may need to be performed to find the correct area. A successful diagnostic block will lead to significant or complete resolution of symptoms for the duration of the anesthetic, and the pattern of numbness should reflect the distribution of symptoms.
Imaging studies
MR and ultrasound can be used for peripheral nerve imaging.[29] Ultrasound is common for superficial nerves of the upper extremity such as carpal tunnel syndrome.[30] MR imaging is not always reliable in that often the clinical assessment and imaging do not match for peripheral neuropathies.[31] That is, there are false positives and false negatives which bring into question how reliable these scans are for diagnosis and surgical planning. There are known limitations of MR for the identification of nerve entrapment:
- Resolution limitations: Small nerves are fairly resistant to imaging and even structures like the sacrococcygeal plexus can't be seen with MR tractography.
- Dynamic nature of entrapment: Nerve entrapment can be dynamic where the symptoms can only be elicited with certain movements. MR imaging is done while the patient is lying still and may not be able to reproduce the conditions of entrapment.
- Focus on structural abnormalities: Nerve entrapment can sometimes result from problems that don't cause visual changes, such as inflammation or the tightness of surrounding tissues.
- Positional limitations: MRIs are done with the patient lying down. The geometry of the machine does not provide room for the patient to sit or stand during the scan where the symptoms may be reproducible. While sitting and standing MRIs exist, the resolution provided is significantly lower (0.6T vs 3.0T).
- Poor visibility of entrapping tissue types: MR visualizes soft tissue according to water content. Tissue types with low water content such as fibrotic tissue are resistant to imaging and yet may be highly clinically significant.
Despite these limitations, MR imaging studies can rule out certain causes of entrapment such as a mass lesion. Increasingly used are specialized forms of MRI such as MR neurography[32]</ref> and MR tractography. Of the two MRT is more effective as it has a high correlation with intraoperative findings.[33]
Electrophysiology studies
The main electrophysiological studies are the nerve conduction study (NCS) and electromyography (EMG). The benefit of nerve conduction studies has not been proven beyond distal entrapment neuropathies (carpal tunnel syndrome and cubital tunnel syndrome).[34] An EMG is limited to just providing information on motor nerves, and provides limited information on the location, extent, and etiology of nerve injury. Electrophysiology is not very useful in pelvic sensory neuropathies or for interrogation of the deep pelvic nerves.[32]
The major limitation of extra-operative electrophysiology studies is that they do not have direct access to the nerve. In contrast, intra-operative electrophysiology studies can be done with direct access to the nerve, and this is a useful tool for nerve decompression surgery. During surgery the studies can be used to identify which nerves innervate given myotomes, identify which blood vessels are essential for a nerve, and to compare nerve conduction before and after decompression.
Treatment
When an underlying medical condition is causing the neuropathy, treatment should first be directed at this condition. Several systemic conditions have been implicated in the development of nerve compression syndromes, including diabetes, thyroid disease, heavy alcohol use, generalized edema, and systemic inflammatory disease.[21] There is substantial evidence to support an association between certain work activities and carpal tunnel syndrome that involve repetitive motion.[35] Certain recreational activities such as bicycling are associated with pudendal neuralgia due to increased pressure on Alcock's canal. [36]
Non-surgical treatments includes rest and activity modification, physical therapy, ergonomic modifications, pain management, and steroid blocks. About 50% of the time, symptoms will improve only conservative measures.[37][38] Opioids can provide short-term pain relief in highly selected patients.[39] Steroid blocks can have a short-term benefit but have not shown to have long-term therapeutic benefit.[40][41]
In select cases botox injections may also be an effective option, such as piriformis syndrome or migraines.[42][43][44] The effectiveness of botox injections is predicated on muscular entrapment such that atrophying a muscle reduces pressure on a nerve.
The decision to proceed with surgical interventions is a matter of when the severity of subjective symptoms outweighs the potential risks and complications. With muscle wasting or electromyographic evidence of denervation, timely surgical decompression is clearly indicated.[21]
Nerve decompression
Nerve decompressions aim to surgically access and explore some segment of nerve, removing any tissue that may be causing compression. In this way a nerve decompression can directly address the underlying cause of entrapment. A nerve decompression can either can be done by open surgery or laparoscopic surgery. In some cases, like carpal tunnel syndrome, either approach is viable.[45] For deeper nerves, a laparoscopic approach is the only choice. New laparoscopic techniques allow surgeons to get access to previously unreachable pelvic structures such as the sacral plexus.[46] Nerve decompressions and resections are the only treatments with a known cure rate. It is a common clinical experience, that even chronic entrapments with longstanding muscle weakness and sensory disturbances sometimes show a very rapid reversibility of some or all of the symptoms after surgical decompression of the nerve.[20]
A large number of nerve decompression surgeries achieve 25+% cure rate, and 75+% success rate.[46][47][48][49] It is not known why separate surgeries would have similar outcomes.
Nerve Resection
Nerve resections aim to eliminate the dermatome entirely along with any positive sensory symptoms such as pain. While nerve decompression may be used on any nerve, nerve resection should only be used on purely sensory nerves when the loss of sensation is acceptable. The superior cluneal nerves, middle cluneal nerves, posterior femoral cutaneous nerve, lateral femoral cutaneous nerve are all sensory and resection may simply be a more "complete" option, as nerve decompressions can't explore every part of the nerve and may miss some entrapment points. Outcomes for nerve resection is similar to nerve decompression.[50][51] One disadvantage of nerve resections is that traumatic injury to the nerve is unavoidable, and a neuroma may form at the point of resection. There are surgical approaches to prevent neuroma formation[52] such as targeted muscle reinnervation[53] which have shown very good results, however the risk of neuroma formation is not completely eliminated.
Neuromodulation
Other surgical treatments include general neuromodulation treatments. Neuromodulation is symptomatic treatment and does not attempt to address the root cause of compression, but rather to alter the signals sent along the nerves to the brain. It can be a suitable choice when the source of compression has been removed, but the positive sensory symptoms such as pain aren't fully resolved. If neuromodulation is used without removing the source of compression, tissue injury might progress leading to worse outcomes when the source of compression is eventually removed. Better known neuromodulation treatments include the spinal cord stimulator and the intrathecal catheter. The disadvantage of these treatments is that they are not targeted for peripheral nerves (implantation is typically in the spinal cord), can only address sensory symptoms, can expose unrelated nerves to injury during implantation if placed in the spine, and have a high failure rate due to device migration. The spinal cord stimulator in particular has a very high complication rate, as high as 40%.[54] Advancements have been made to move these devices closer to peripheral nerves such as peripheral nerve stimulation[55] and the peripheral nerve catheter.[56] A challenge with these new treatment is that peripheral nerves are highly mobile, and it is difficult to fix a wire or tube to something that's constantly moving.
See also
References
- "Nerve Entrapment Syndromes: Background, History of the Procedure, Problem". 2018-05-23.
{{cite journal}}: Cite journal requires|journal=(help) - Aoun F, Alkassis M, Tayeh GA, Chebel JA, Semaan A, Sarkis J, Mansour R, Mjaess G, Albisinni S, Absil F, Bollens R, Roumeguère T. Sexual dysfunction due to pudendal neuralgia: a systematic review. Transl Androl Urol. 2021 Jun;10(6):2500-2511. doi:10.21037/tau-21-13. PMID 34295736; PMCID: PMC8261452.
- Alimehmeti RH, Schuenke MD, Dellon AL. Anococcygeal Nerve and Sitting Pain: Differential Diagnosis and Treatment Results. Ann Plast Surg. 2022 Jan 1;88(1):79-83. doi:10.1097/SAP.0000000000002920. PMID 34670963.
- Dellon AL. Pain with sitting related to injury of the posterior femoral cutaneous nerve. Microsurgery. 2015 Sep;35(6):463-8. doi:10.1002/micr.22422. Epub 2015 Apr 27. PMID 25917688.
- Kaur J, Leslie SW, Singh P. Pudendal Nerve Entrapment Syndrome. [Updated 2022 Nov 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544272/
- Latef TJ, Bilal M, Vetter M, Iwanaga J, Oskouian RJ, Tubbs RS. Injury of the Radial Nerve in the Arm: A Review. Cureus. 2018 Feb 16;10(2):e2199. doi:10.7759/cureus.2199. PMID: 29666777; PMCID: PMC5902095.
 This article incorporates text from this source, which is available under the CC BY 3.0 license.
This article incorporates text from this source, which is available under the CC BY 3.0 license. - Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005 Aug;33(8):1224-30. doi:10.1177/0363546505275131. Epub 2005 Jul 6. PMID: 16000656.
- Jackson JA, Olsson D, Burdorf A, et al Occupational biomechanical risk factors for radial nerve entrapment in a 13-year prospective study among male construction workers Occupational and Environmental Medicine 2019;76:326-331.
- Yu JK, Yang JS, Kang SH, Cho YJ. Clinical characteristics of peroneal nerve palsy by posture. J Korean Neurosurg Soc. 2013 May;53(5):269-73. doi:10.3340/jkns.2013.53.5.269. Epub 2013 May 31. PMID: 23908699; PMCID: PMC3730027.
- Martin R, Martin HD, Kivlan BR. NERVE ENTRAPMENT IN THE HIP REGION: CURRENT CONCEPTS REVIEW. Int J Sports Phys Ther. 2017 Dec;12(7):1163-1173. doi:10.26603/ijspt20171163. PMID: 29234567; PMCID: PMC5717491.
- Kennedy J. Neurologic injuries in cycling and bike riding. Neurol Clin. 2008 Feb;26(1):271-9; xi-xii. doi:10.1016/j.ncl.2007.11.001. PMID: 18295095.
- Rota E, Morelli N. Entrapment neuropathies in diabetes mellitus. World J Diabetes. 2016 Sep 15;7(17):342-53. doi:10.4239/wjd.v7.i17.342. PMID: 27660694; PMCID: PMC5027001.
- Hatem, Munif & Martin, Hal. (2022). Deep Gluteal Space with Surgical Technique. 10.1007/978-3-030-43240-9_75.
- Shoji K, Heng M, Harris MB, Appleton PT, Vrahas MS, Weaver MJ. Time From Injury to Surgical Fixation of Diaphyseal Humerus Fractures Is Not Associated With an Increased Risk of Iatrogenic Radial Nerve Palsy. J Orthop Trauma. 2017 Sep;31(9):491-496. doi:10.1097/BOT.0000000000000875. PMID: 28459772.
- Muniz Neto FJ, Kihara Filho EN, Miranda FC, Rosemberg LA, Santos DCB, Taneja AK. Demystifying MR Neurography of the Lumbosacral Plexus: From Protocols to Pathologies. Biomed Res Int. 2018 Jan 31;2018:9608947. doi:10.1155/2018/9608947. PMID: 29662907; PMCID: PMC5832061.
- Park MS, Yoon SJ, Jung SY, Kim SH. Clinical results of endoscopic sciatic nerve decompression for deep gluteal syndrome: mean 2-year follow-up. BMC Musculoskelet Disord. 2016 May 20;17:218. doi:10.1186/s12891-016-1062-3. PMID: 27206482; PMCID: PMC4875686.
- Metikala S, Sharma V. Endoscopic Sciatic Neurolysis for Deep Gluteal Syndrome: A Systematic Review. Cureus. 2022 Mar 14;14(3):e23153. doi:10.7759/cureus.23153. PMID: 35444897; PMCID: PMC9010003.
- Genova A, Dix O, Saefan A, Thakur M, Hassan A. Carpal Tunnel Syndrome: A Review of Literature. Cureus. 2020 Mar 19;12(3):e7333. doi:10.7759/cureus.7333. PMID: 32313774; PMCID: PMC7164699.
- Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Künnapuu K, Mägi R, Bennett DL, Furniss D. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat Commun. 2019 Mar 4;10(1):1030. doi:10.1038/s41467-019-08993-6. PMID: 30833571; PMCID: PMC6399342.
- Thatte MR, Mansukhani KA. Compressive neuropathy in the upper limb. Indian J Plast Surg. 2011 May;44(2):283-97. doi:10.4103/0970-0358.85350. PMID 22022039; PMCID: PMC3193641.
- Tang, David T. M.D.; Barbour, John R. M.D.; Davidge, Kristen M. M.D.; Yee, Andrew B.Sc.; Mackinnon, Susan E. M.D.. Nerve Entrapment: Update. Plastic and Reconstructive Surgery 135(1):p 199e-215e, January 2015. doi:10.1097/PRS.0000000000000828
- Schmid AB, Fundaun J, Tampin B. Entrapment neuropathies: a contemporary approach to pathophysiology, clinical assessment, and management. Pain Rep. 2020 Jul 22;5(4):e829. doi:10.1097/PR9.0000000000000829. PMID 32766466; PMCID: PMC7382548.
- Mackinnon SE. Pathophysiology of nerve compression. Hand Clin. 2002 May;18(2):231-41. doi:10.1016/s0749-0712(01)00012-9. PMID 12371026.
- National Research Council (US) Steering Committee for the Workshop on Work-Related Musculoskeletal Injuries: The Research Base. Work-Related Musculoskeletal Disorders: Report, Workshop Summary, and Workshop Papers. Washington (DC): National Academies Press (US); 1999. Biological Response of Peripheral Nerves to Loading: Pathophysiology of Nerve Compression Syndromes and Vibration Induced Neuropathy. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230871/
- Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27(4):306-10. doi:10.1002/nau.20505. PMID 17828787.
- Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand. 2008 Jul;52(6):727-37. doi:10.1111/j.1399-6576.2008.01666.x. Epub 2008 May 12. PMID 18477070.
- Wadhwa V, Scott KM, Rozen S, Starr AJ, Chhabra A. CT-guided Perineural Injections for Chronic Pelvic Pain. Radiographics. 2016 Sep-Oct;36(5):1408-25. doi:10.1148/rg.2016150263. PMID 27618322.
- Fritz J, Chhabra A, Wang KC, Carrino JA. Magnetic resonance neurography-guided nerve blocks for the diagnosis and treatment of chronic pelvic pain syndrome. Neuroimaging Clin N Am. 2014 Feb;24(1):211-34. doi:10.1016/j.nic.2013.03.028. Epub 2013 Aug 1. PMID 24210321.
- Stoll G, Wilder-Smith E, Bendszus M. Imaging of the peripheral nervous system. Handb Clin Neurol. 2013;115:137-53. doi:10.1016/B978-0-444-52902-2.00008-4. PMID 23931778.
- Ng AWH, Griffith JF, Lee RKL, Tse WL, Wong CWY, Ho PC. Ultrasound carpal tunnel syndrome: additional criteria for diagnosis. Clin Radiol. 2018 Feb;73(2):214.e11-214.e18. doi:10.1016/j.crad.2017.07.025. Epub 2017 Aug 30. PMID 28859853.
- van Rijn JC, Klemetso N, Reitsma JB, Majoie CB, Hulsmans FJ, Peul WC, Bossuyt PM, Heeten GJ, Stam J. Symptomatic and asymptomatic abnormalities in patients with lumbosacral radicular syndrome: Clinical examination compared with MRI. Clin Neurol Neurosurg. 2006 Sep;108(6):553-7. doi:10.1016/j.clineuro.2005.10.003. Epub 2005 Nov 10. PMID 16289310.
- Weissman, Eric; Boothe, Ethan; Wadhwa, Vibhor; Scott, Kelly; Chhabra, Avneesh (1 June 2017). "Magnetic Resonance Neurography of the Pelvic Nerves". Seminars in Ultrasound, CT and MRI. 38 (3): 269–278. doi:10.1053/j.sult.2016.11.006. ISSN 0887-2171.
- Lemos N, Melo HJF, Sermer C, Fernandes G, Ribeiro A, Nascimento G, Luo ZC, Girão MJBC, Goldman SM. Lumbosacral plexus MR tractography: A novel diagnostic tool for extraspinal sciatica and pudendal neuralgia? Magn Reson Imaging. 2021 Nov;83:107-113. doi:10.1016/j.mri.2021.08.003. Epub 2021 Aug 14. PMID 34400289.
- Cho SC, Ferrante MA, Levin KH, Harmon RL, So YT. Utility of electrodiagnostic testing in evaluating patients with lumbosacral radiculopathy: An evidence-based review. Muscle Nerve. 2010 Aug;42(2):276-82. doi:10.1002/mus.21759. PMID 20658602.
- Latko WA, Armstrong TJ, Franzblau A, Ulin SS, Werner RA, Albers JW. Cross-sectional study of the relationship between repetitive work and the prevalence of upper limb musculoskeletal disorders. Am J Ind Med. 1999 Aug;36(2):248-59. doi:10.1002/(sici)1097-0274(199908)36:2<248::aid-ajim4>3.0.co;2-q. PMID 10398933.
- Leibovitch I, Mor Y. The vicious cycling: bicycling related urogenital disorders. Eur Urol. 2005 Mar;47(3):277-86; discussion 286-7. doi:10.1016/j.eururo.2004.10.024. Epub 2004 Dec 30. PMID 15716187.
- Ortiz-Corredor F, Enríquez F, Díaz-Ruíz J, Calambas N. Natural evolution of carpal tunnel syndrome in untreated patients. Clin Neurophysiol. 2008 Jun;119(6):1373-8. doi:10.1016/j.clinph.2008.02.012. Epub 2008 Apr 18. PMID 18396098.
- Konstantinou K, Dunn KM, Ogollah R, Lewis M, van der Windt D, Hay EM; ATLAS Study Team. Prognosis of sciatica and back-related leg pain in primary care: the ATLAS cohort. Spine J. 2018 Jun;18(6):1030-1040. doi:10.1016/j.spinee.2017.10.071. Epub 2017 Nov 21. PMID 29174459; PMCID: PMC5984249.
- Sommer C, Klose P, Welsch P, Petzke F, Häuser W. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Eur J Pain. 2020 Jan;24(1):3-18. doi:10.1002/ejp.1494. Epub 2019 Nov 18. PMID 31705717.
- Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, Koes B, Ferreira PH. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med. 2012 Dec 18;157(12):865-77. doi:10.7326/0003-4819-157-12-201212180-00564. PMID 23362516.
- Labat JJ, Riant T, Lassaux A, Rioult B, Rabischong B, Khalfallah M, Volteau C, Leroi AM, Ploteau S. Adding corticosteroids to the pudendal nerve block for pudendal neuralgia: a randomised, double-blind, controlled trial. BJOG. 2017 Jan;124(2):251-260. doi:10.1111/1471-0528.14222. Epub 2016 Jul 27. PMID 27465823; PMCID: PMC5215631.
- Lang AM. Botulinum toxin type B in piriformis syndrome. Am J Phys Med Rehabil. 2004 Mar;83(3):198-202. doi: 10.1097/01.phm.0000113404.35647.d8. PMID: 15043354.
- Yan K, Xi Y, Hlis R, Chhabra A. Piriformis syndrome: pain response outcomes following CT-guided injection and incremental value of botulinum toxin injection. Diagn Interv Radiol. 2021 Jan;27(1):126-133. doi: 10.5152/dir.2020.19444. PMID: 33252337; PMCID: PMC7837716.
- Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, Diener HC, Brin MF; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010 Jul;30(7):793-803. doi: 10.1177/0333102410364676. Epub 2010 Mar 17. PMID: 20647170.
- Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007 Oct 17;2007(4):CD003905. doi:10.1002/14651858.CD003905.pub3. PMID 17943805; PMCID: PMC6823225.
- Lemos N, Possover M. Laparoscopic approach to intrapelvic nerve entrapments. J Hip Preserv Surg. 2015 Jul;2(2):92-8. doi:10.1093/jhps/hnv030. Epub 2015 Jun 6. PMID 27011825; PMCID: PMC4718483.
- Martin HD, Shears SA, Johnson JC, Smathers AM, Palmer IJ. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011 Feb;27(2):172-81. doi:10.1016/j.arthro.2010.07.008. Epub 2010 Nov 11. PMID 21071168.
- Jottard K, Bruyninx L, Bonnet P, De Wachter S. Endoscopic trans gluteal minimal-invasive approach for nerve liberation (ENTRAMI technique) in case of pudendal and/or cluneal neuralgia by entrapment: One-year follow-up. Neurourol Urodyn. 2020 Sep;39(7):2003-2007. doi:10.1002/nau.24462. Epub 2020 Jul 17. PMID 32678485.
- ElHawary H, Barone N, Baradaran A, Janis JE. Efficacy and Safety of Migraine Surgery: A Systematic Review and Meta-analysis of Outcomes and Complication Rates. Ann Surg. 2022 Feb 1;275(2):e315-e323. doi:10.1097/SLA.0000000000005057. PMID 35007230.
- Dellon AL. Pain with sitting related to injury of the posterior femoral cutaneous nerve. Microsurgery. 2015 Sep;35(6):463-8. doi:10.1002/micr.22422. Epub 2015 Apr 27. PMID 25917688.
- Zacest AC, Magill ST, Anderson VC, Burchiel KJ. Long-term outcome following ilioinguinal neurectomy for chronic pain. J Neurosurg. 2010 Apr;112(4):784-9. doi:10.3171/2009.8.JNS09533. PMID 19780646.
- Scott BB, Winograd JM, Redmond RW. Surgical Approaches for Prevention of Neuroma at Time of Peripheral Nerve Injury. Front Surg. 2022 Jun 27;9:819608. doi: 10.3389/fsurg.2022.819608. PMID: 35832494; PMCID: PMC9271873.
- Valerio IL, Dumanian GA, Jordan SW, Mioton LM, Bowen JB, West JM, Porter K, Ko JH, Souza JM, Potter BK. Preemptive Treatment of Phantom and Residual Limb Pain with Targeted Muscle Reinnervation at the Time of Major Limb Amputation. J Am Coll Surg. 2019 Mar;228(3):217-226. doi: 10.1016/j.jamcollsurg.2018.12.015. Epub 2019 Jan 8. PMID: 30634038.
- Shim JH. Limitations of spinal cord stimulation for pain management. Korean J Anesthesiol. 2015 Aug;68(4):321-2. doi:10.4097/kjae.2015.68.4.321. PMID 26257842; PMCID: PMC4524928.
- Helm, S., Shirsat, N., Calodney, A. et al. Peripheral Nerve Stimulation for Chronic Pain: A Systematic Review of Effectiveness and Safety. Pain Ther 10, 985–1002 (2021). doi:10.1007/s40122-021-00306-4
- Soffin, Ellen M. MD, PhD; YaDeau, Jacques T. MD, PhD. Peripheral Nerve Catheters: Ready for a Central Role?. Anesthesia & Analgesia 124(1):p 4-6, January 2017. | doi:10.1213/ANE.0000000000001642